In Situ Growth of g-C3N4 on Hexangular Flowerlike FeWO4 Microcrystals: Highly Efficient Catalyst and the Crucial Roles of Fe3+/Fe2+ Couple in the Photoassisted Oxidation and Reduction Reactions
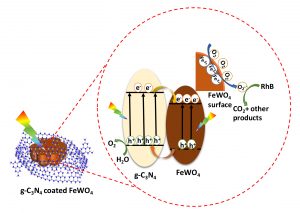
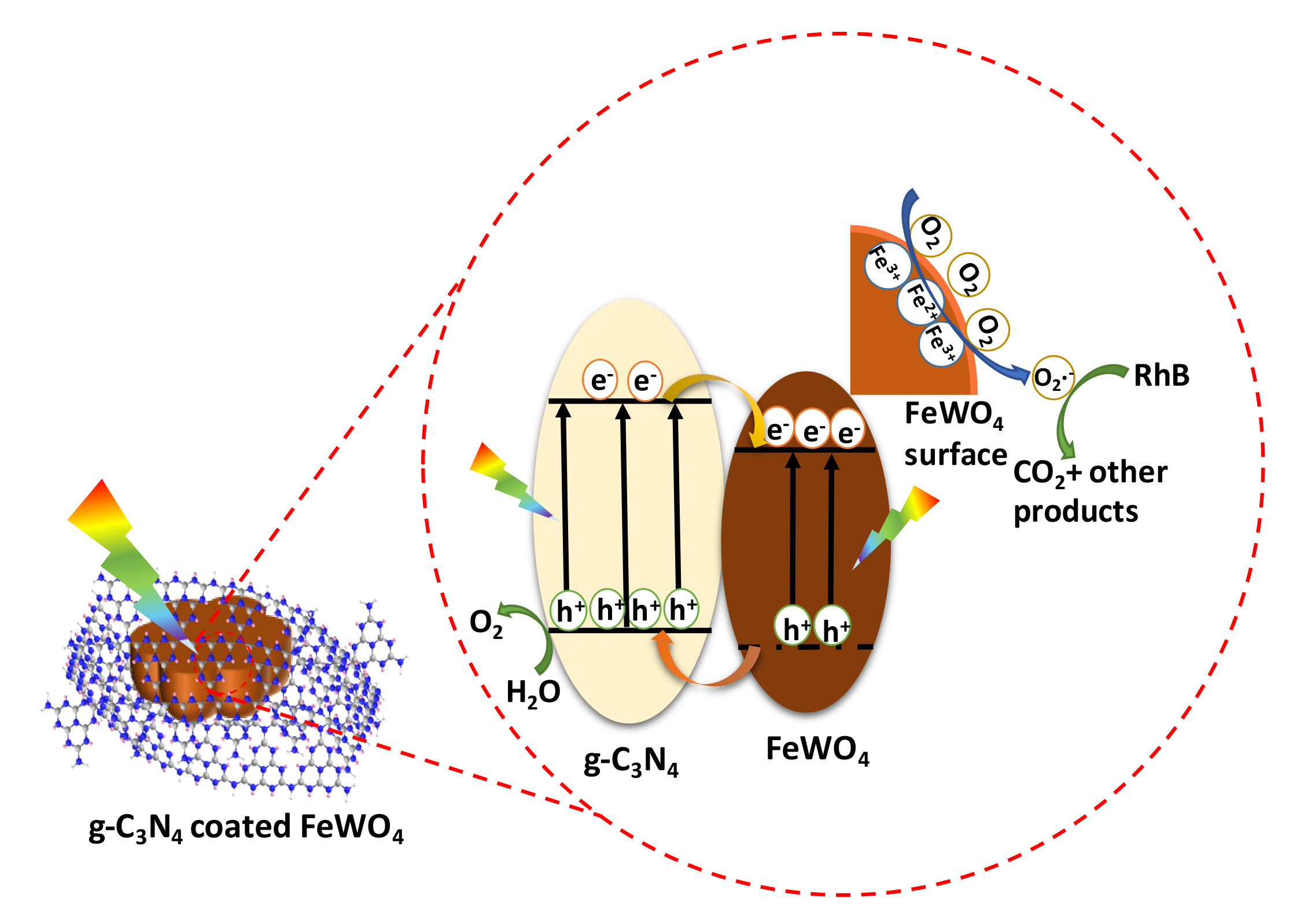
Novel highly efficient heterostructured photocatalysts consisting of g-C3N4 nanosheets on hexangular flowerlike FeWO4 microcrystals were first fabricated via an in situ growth approach. The intimate g-C3N4 coating layers (ca. 50–130 nm) on the surface of FeWO4 microcrystals were observed through transmission electron microscopy. An all-side evaluation of its performance toward typical photoassisted oxidation and reduction reactions was carried out, which exhibited drastically improved performance for photocatalytic oxygen production and photodegradation, while obviously suppressed hydrogen evolution activity when compared with the bulk g-C3N4(CNU). The optimal photocatalyst CNU–FW90 (90 mg of FeWO4 in 15 g of urea) displayed an oxygen evolution rate of 303 μmol/(g h), about 2.4 and 3.8 times as much as that of CNU and FeWO4, respectively. Not only that, CNU–FW90 showed superior degradation activity with a reaction kinetics rate constant of 0.1192 min–1, nearly 5-fold higher than that of the pure CNU. By correlating the performance with the band structures and surface valence states of g-C3N4@FeWO4 heterojunctions, a possible mechanism was proposed to uncover the differences between oxidation and reduction reactions in this system, which highlighted the crucial role of Fe3+/Fe2+ couple in tuning the performance of photocatalytic reactions. This work put forward a promising strategy to design highly efficient semiconductor photocatalysts and shed light on the vital effect of surface valence state of Fe element in tuning the performance of Fe-related photocatalysts.
J. Phys. Chem. C122, 24, 12900-12912
 Wei-Lin Dai Group
Wei-Lin Dai Group